Why Live Vaccines Are More Effective
A research team from Charité - Universitätsmedizin Berlin and Freie Universität Berlin has investigated the immune response to different vaccinations.
Sep 17, 2018
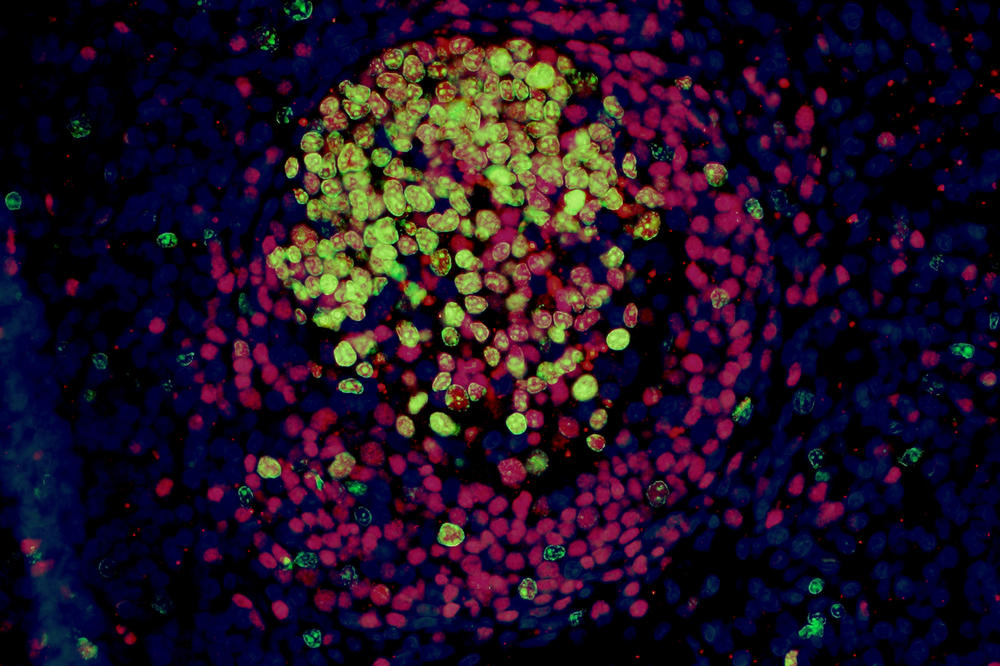
The image shows an immunofluorescence of a lymph follicle with active germ center after successful vaccination with live vaccine
Image Credit: Kristina Dietert, Freie Universität Berlin
The first human patient was vaccinated in 1796. Edward Jenner, an English country doctor, infected a boy with harmless cowpox, thus protecting him from the then rampant and usually fatal smallpox. “Active immunization” was invented and led to the development of numerous successful vaccines.
In addition to live vaccines, such as the one against the now extinct smallpox, which are based on similar, or severely weakened, so-called attenuated viruses or bacteria, there are also vaccines inactivated by heat or in other ways. Vaccinations with the latter are considered to be less risky, but often need to be refreshed regularly. “Satisfactory immunization is often only achieved after three vaccinations, for example with the hepatitis vaccine,” says Leif Erik Sander, infection physician at Charité - Universitätsmedizin Berlin. Only additives, so-called adjuvants, such as the aluminum salts often vilified by vaccination opponents, turn them into acceptable vaccines. Conversely, a single live vaccination against measles or rubella protects over 95 percent of those vaccinated – usually for life.
Leif Erik Sander is an infection physician at Charité - Universitätsmedizin Berlin.
Image Credit: Prof. Dr. Leif Sander, Berlin
“We have made the fundamental discovery that the immune system takes an intruder more seriously when the microorganism still contains RNA molecules.” Leif Erik Sander
The reasons why this difference in effect exists were unknown up to now. A few years ago, Leif Erik Sander observed that certain immune cells in mice were able to recognize whether a pathogen was dead or alive. “At that time, we made the fundamental discovery that when microorganisms, such as bacteria, still contain RNA molecules and thus a copy of their genetic information, the immune system takes the intruder much more “seriously.” If, on the other hand, the microorganisms are recognized as dead, the defense only activates its saving mode.” The team of researchers led by Sander has now been able to elucidate the molecular basis of this recognition process. “We first asked ourselves whether this also works in humans and if so, via which receptors?” The researchers infected human cells in test tubes and were successful with phagosomes in scavenger cells. “Pathogens are usually chopped up in these cell organelles. They then present the corresponding antigens and induce the aggravated, so-called adaptive immune defense.” This long-lasting immune response requires certain helper cells. However, these only appear on the scene when a certain cell “checkpoint” – the Toll-like receptor 8 (TLR8) – sounds the alarm. And this is only activated by living pathogens or their RNA, respectively. Using certain messenger substances, follicular helper cells (TFH cells) are now being called onto the scene to help form effective antibodies that kill the intruder.
“If we had done the experiments on mice, we wouldn’t have figured it out,” explains Sander. “Mice also possess the TLR8 but, because by a whim of nature, it doesn’t work on them as it does on most other species.” Instead of researching on sterile animals in the laboratory, Sander and his team decided to work with farm animals. To be specific: with pigs from breeding farms that are already routinely vaccinated against salmonella infections, for example.
Friederike Ebner is a postdoctoral researcher in the Department of Veterinary Medicine at Freie Universität Berlin.
Image Credit: Institut für Immunologie der Freien Universität Berlin
“This exciting project has taught us a lot.” Friederike Ebner
This was where the veterinarians at Freie Universität Berlin came into play. While Achim Gruber and Kristina Dietert at the Institute of Veterinary Pathology at Freie Universität used microscopic tissue analysis to study the effect of different vaccine variants on the organism, their colleagues at the Institute of Immunology at Freie Universität examined the TLR8-mediated immune responses in pigs. Using fluorescence-labeled cell sorting, Susanne Hartmann and Friederike Ebner isolated certain immune cells from the pig spleen and, together with their Charité colleagues, established the first corresponding in vitro test.
In short: Sander’s findings on human cells were also confirmed in pigs. There were clear differences in the immune response, depending on whether the cells were stimulated with living or inactivated pathogens. “We learned a lot on this exciting project,” says Friederike Ebner, a postdoctoral researcher at the Department of Veterinary Medicine at Freie Universität Berlin. “And the data have once again convinced us to use pigs as model organisms for humans in infection immunology.”
Leif Erik Sander and his team are already working on the next step, namely the development of novel additives for inactivated vaccines that trigger the same alarm at checkpoint TLR8 as the RNA of living pathogens. “As the vaccines would then have to be cooled constantly, RNA itself might be too unstable for this purpose. We are therefore looking for small molecules that resemble building blocks of nucleic acids.” There are already some promising candidates.