Learning from the Mealworm Beetle's Immune System
Reserachers from Freie Universität Berlin and Charité – Universitätsmedizin Berlin are working on a device that will help predict the development of antibiotic resistance in bacteria.
Mar 16, 2018
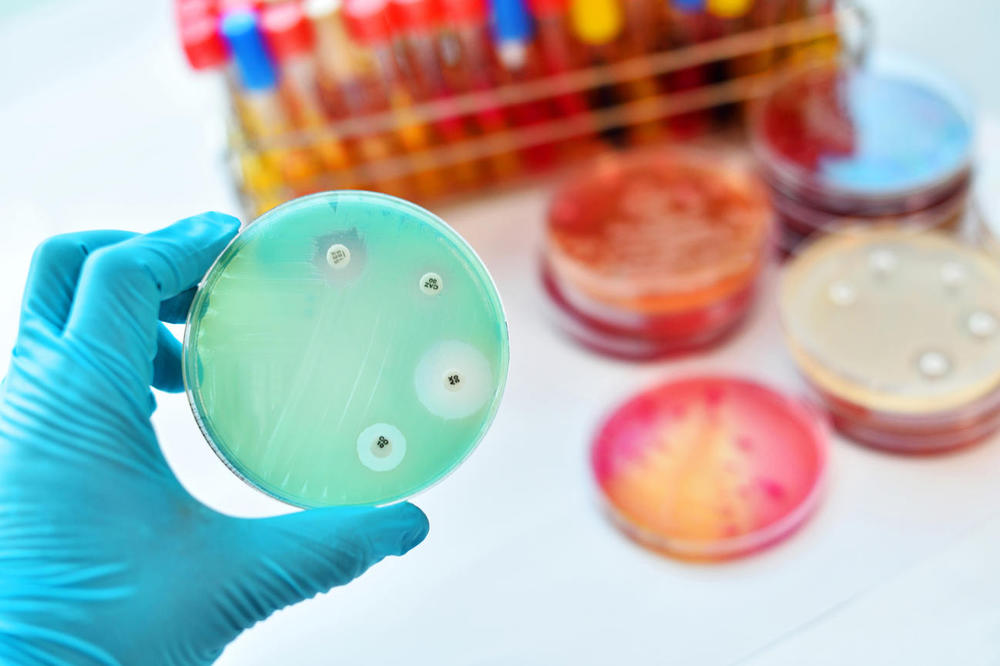
Four drops of different antibiotics were applied to a bacterial lawn dyed green. The white ring around each one varies in thickness, showing how effective the antibiotic is. The white spots are where the bacteria have been killed.
Image Credit: istockphoto - R. Naylor
Jens Rolff, a professor of evolutionary biology at the Institute of Biology at Freie Universität Berlin, observes the enemy at work and compares various counter-strategies in an experiment.
“On average, it only takes two years for the first resistance to a new antibiotic to emerge,” Rolff says. That means there is little incentive for drug companies to develop new medications. Through an approach known as “antibiotic stewardship” – management of antibiotic use – medical centers are trying to get the issue under control. They are developing plans for selection, dosage, use, and combination of antibiotics in order to achieve the best results, with minimal side effects and at as little cost as possible.
In the process, they are relying first and foremost on past experience, since there has thus far been no scientific method they can use to predict the formation of resistance. “Basically, how resistance develops has not yet been studied closely,” Rolff explains. “Evolve” is the term of art here, since resistant pathogens spread chiefly through the process of evolution – genetic changes and selection that take place over generations.
Strains of bacteria whose genes make them impervious to certain medications have existed for a long time, and random genetic changes mean that new ones are being added all the time. However, the widespread use of antibiotics in people and animals often kills off strains that do respond to treatment, without there being sufficient reason to do so. This means the bacteria that carry the genes for drug resistance can multiply without competition. Rolff says medical professionals often lose sight of this mechanism of selection.
All that is about to change now. Together with Ulrich Kertzscher and Klaus Affeld of the Biofluid Mechanics Lab at Charité – Universitätsmedizin Berlin, Rolff is working on a compact lab device called “Evol-Chip” that can be used to track how various antibiotics affect the evolution of bacteria in experimental terms. The European Research Council (ERC) awarded the project its prestigious Proof of Concept Grant, which comes with 150,000 euros in funding.
“Within just a few weeks we simulate what takes several years in a natural environment,” Rolff explains. “We want to observe, compare, and ultimately predict how resistance arises under different conditions.” EvolChip, he says, gives medical professionals who work in a clinical lab setting their first-ever chance to stay one step ahead of evolution: Instead of the existing process, in which tests are performed to see what kinds of drug resistance the bacteria infecting a patient have already developed, they could find out which active ingredients, combinations, and dosages will slow the process of genetic adaptation.
Evolutionary biologist Jens Rolff does research on the complex immune system of insects.
Image Credit: Bernd Wannenmacher
A micropump is used to add antibiotic in ultrafine dosages and with precision targeting, to a bacterial culture that is growing in a nutrient solution at a speed of 20 to 60 minutes per generation. The microorganisms have been marked with fluorescent dye, making it easy to see where they have not been killed off by the medications, but instead are continuing to multiply. “We can take a sample of resistant bacteria with a syringe, sequence the genome, and see what genetic changes they show,” Rolff says.
Conditions in the culture medium are different from those inside a patient’s body, but what the researchers are especially interested in is the comparative effects of different sequences, concentrations, and combinations of antibiotics. “A comparative prediction based on a scientific model that has been tested is much better than no prediction at all,” the professor explains, adding that the model could help clinical staff decide on which antibiotics and dosages to use while also advancing research in this field.
Rolff’s actual field of interest is the complex immune system of insects. Bugs, flies, and butterflies produce dozens of different antimicrobial peptides (AMPs) at once to protect themselves against bacteria. In humans, AMPs are found on the skin and in the mucous membranes, among other places. These chains of amino acids have already been discussed in both human and veterinary medicine as a new silver bullet against microorganisms, but their promise has so far gone unfulfilled.
Rolff believes greater promise lies in analyzing the overall concept of immune defense in insects and learning from it: “The mealworm beetle produces a whole cocktail of defensive agents as needed. It’s very labor-intensive for its metabolism, but it works. In human medicine, you might think of it as being like the kinds of combination therapy that are already used to fight viruses like HIV.”
In his “Evoresin” research project, which has also received support from the European Research Council (ERC), Rolff compared the effects of AMP cocktails on bacteria against those of antibiotics. To simulate the evolutionary “race” under true-to-life conditions and observe it, he worked with engineers from Charité to create the predecessor to the EvolChip device
Rolff is consulting with practicing medical professionals and veterinarians to figure out how EvolChip can be optimized for everyday clinical use. Thanks to the funding the project has received, a postdoc has been brought on board full-time to work on further development and test runs. The business model and sales concept are being developed by the researchers together with the team from Profund Innovation, the service institution for knowledge and technology transfer within the research division of Freie Universität.
The goal is to produce a market-ready device, which will first be tested at a university medical center. Still, researcher Jens Rolff does not wish to become an entrepreneur. He’s happy to leave that side of things to colleagues who have more enthusiasm for it. “But I’d be delighted if our idea does end up being used in practice,” he says.
Further Information
Prof. Dr. Jens Rolff, Professor of Evolutionary Biology, Institute of Biology, Freie Universität Berlin, Tel.: +49 30 838-54893, Email: jens.rolff@fu-berlin.de