The Natural Velcro Fastener
Berlin researchers are investigating the principle of multivalence with a view to using it in medical and biotechnology applications.
Sep 08, 2017
Multivalence at play: when a gecko runs across glass, tiny hairs on its feet interact with the glass.
Image Credit: Skitterphoto/ Pixabay (CC0 Creative Commons)
A gecko effortlessly crosses a smooth glass surface, head downwards. This is multivalence at large. Multivalence is a principle of nature whereby a strong connection arises between numbers of loosely interacting binding partners. In the case of the gecko, these loosely interacting elements are the thousands of tiny hairs on its feet, which enter into a reciprocal action with the glass. The attachment of an individual hair with the surface of the glass may be weak but, as a conglomerate, the hairs allow the gecko a firm grip. This mechanism can be found in many biological processes. Viruses or bacteria use it to attach themselves to the host cell in order to infect it.
Researchers working for the Collaborative Research Center 765 “Multivalency as chemical organization and action principle” were inspired when observing this natural phenomenon and wondered how it might be applied scientifically. Eight Berlin research institutions participate in the Center, which is based at Freie Universität; these include Humboldt Universität zu Berlin, Charité, Technische Universität Berlin and the Robert Koch Institute.
Scanning Inflammation
The multivalent binding macromolecule that goes by the name of “polyglycerol sulphate” inhibits inflammation in painful conditions affecting the joints, such as arthritis or rheumatism. To do this, it attaches itself to inflammatory substances and thereby prevents inflammation. The molecule can do more besides; the synthesis of a fluorescing coloring agent is also based on polyglycerol sulphate. It too binds to the inflammatory substance, which thereby becomes visible with the help of a rheumatism scanner. This allows the illness to be documented in its early stages, which can be decisive when it comes to successful treatment. Both active substances were tested in pre-clinical studies as part of a transfer project involving the firm mivenion GmbH.
The success of the research collaboration is due to its strong base in fundamental research, according to chemistry professor Rainer Haag of Freie Universität, spokesperson for the Collaborative Research Center, which is funded by the German Research Foundation (DFG). “The multivalence project originated in the field of chemistry. During the three funding periods from 2008 to the present, it has proceeded swiftly to application and gone on to development in other disciplines such as biology, biophysics and medicine.” The Leibniz-Humboldt professor, Christian Hackenberger, of the Leibniz-Forschungsinstitut für Molekulare Pharmakologie stresses that one must not lose sight of the underlying mechanisms.
Blocking Viruses
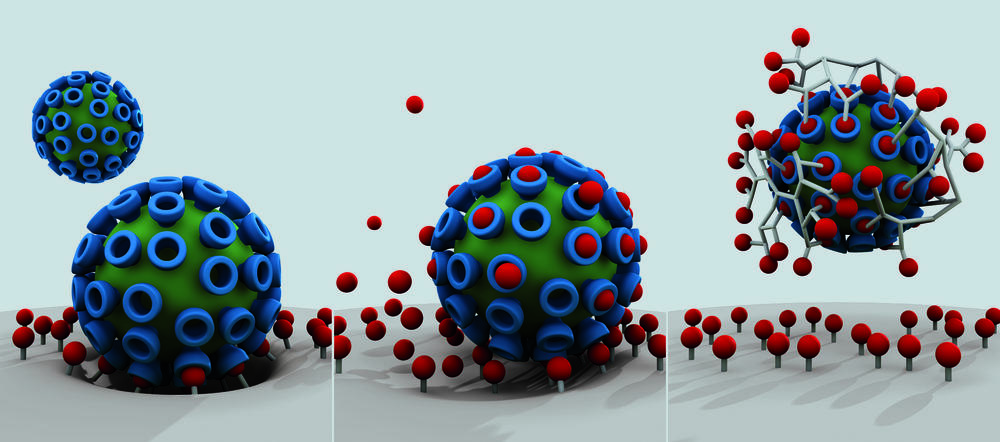
The virus-cell bond as first step in viral infection (r.), with the traditional active substance providing some defense (middle). In contrast, multivalent active substances (r.) effectively screen viruses and block the infection.
Image Credit: Studio Good (T. Päch) / Bearbeitung W. Fischer
Findings from the research into multivalence allow pathogens to be more easily counteracted. It is only fairly recently that scientists participating in the Collaborative Research Center have succeeded in making important advances in developing antiviral medication. A study was organized by four different work groups in the areas of chemistry, biophysics, structural biology, and medicine and published in April 2017 in the scientific journal Angewandte Chemie. The researchers describe how flu viruses can be rendered harmless using upgraded nanosystems. The active substances are short antibody fragments that “hang” from the branches of a tree-like frame. These bond with the viruses and prevent their attachment to and entry into lung cells. This effectively screens the virus. The binding strength of these nanosystems exceeds that of the solitary antibody fragments on the virus severalfold. Haag explains that it has been possible to provide conclusive evidence that similar active substances against other viruses would be a possibility.
The chemist Christian Hackenberger explains that it is important to establish the correct size and constitution of the framework to which the binding partners will be attached. The researchers are working at nanometer level here; for instance, a flu virus has a diameter of roughly 100 nanometers. As a comparison, a pinhead has a diameter of around one million nanometers.
“Each time, the right balance must be found between rigidity and flexibility,” Hackenberger says. He uses the example of a Velcro fastener as an analogy. “The many small hooks on one side of the strip attach themselves to the eyelets on the other side and thus create a very strong bond – although the individual connections between hooks and eyelets are in themselves weak.” A certain pliability of the carrier fabric is needed so that the hooks and eyelets have the play they need in order to find each other. This also applies when it is time for the attachment to be released; the reversibility of the bond is a determining factor. A gecko will not remain permanently on a glass surface.
Developing Bacteria
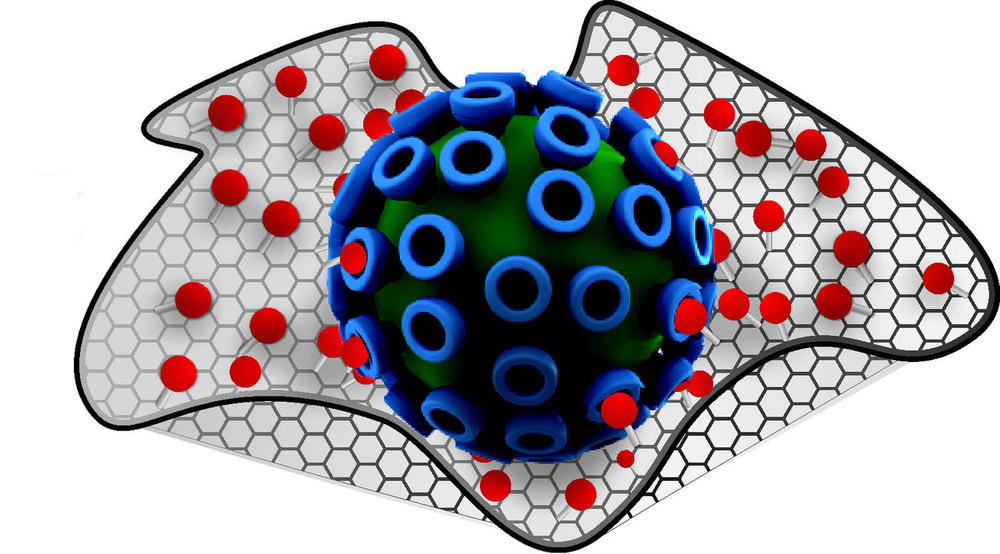
Carbon lattices enfold the germs like tin foil wrapped round a meatball.
Image Credit: Studio Good (T. Päch) / W. Fischer
In order to achieve strong but reversible attachments, Haag’s group is experimenting with graphene as a carrier fabric. This thin, two-dimensional carbon structure is very pliable but also extremely stable. The researchers “decorate” a layer of graphene with molecules, which bond with the surface of the bacteria and at the same time wrap around them. Together with Largentec – a start-up at the campus of Freie Universität – they now hope to build filter systems which will be used to purify bacteria-contaminated water. An absorbent material made of layers of this graphene will catch the bacteria and kill them using oxygen radicals – “catch and kill” is the aptly worded product slogan.
With some satisfaction, the researchers can look back on almost 10 years of the Collaborative Research Center. And, above all, they can be confident about the future. As a result of the intensive cooperation, members of the team have got to know each other really well and established enormous trust, says Hackenberger. “These are people accustomed to hard work.” The project has spread to other research groups with the result that in future the principle of multivalence can be researched at interdisciplinary and transdisciplinary levels.